
•412 patients with histologically proven CHB were enrolled.
•In this prospective multicenter study, adult patients with CHB and valid liver pathological results were recruited to validate the operational and diagnostic performance of a TE device by FibroTouch for staging liver fibrosis.
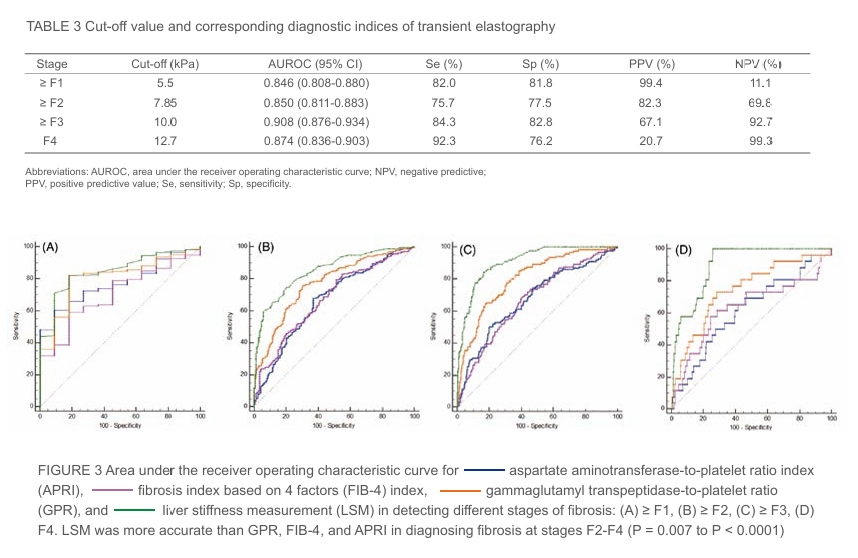
•The area under the receiver operating characteristic curve for the LSM was 0.846 (95% confidence interval [CI] 0.808-0.880) for fibrosis stage ≥ F1, 0.850 (95% CI 0.811-0.883) for ≥ F2, 0.908 (95% CI 0.876-0.934) for ≥ F3 and 0.874 (95% CI 0.836-0.903) for F4.
•The diagnostic accuracy of LSM was superior to that of gamma-glutamyl transpeptidase-to-platelet ratio (GPR), aminotransferase-to-platelet ratio index (APRI), or fibrosis index based on 4 factors (FIB-4) index in staging fibrosis F2-F4 (P = 0.007 to < 0.0001). Optimal LSM cut-off values for diagnosing fibrosis stage ≥ F1, ≥ F2, ≥ F3, and F4 were 5.5 kPa, 7.85 kPa, 10.0 kPa, and 12.7 kPa, respectively.
