
•88 patients with compensated hepatitis B cirrhosis, 38 received entecavir,17 received entecavir combined with PEG-IFNα-2a and 33 received entecavir and thymosin α1 combination for 104 weeks.
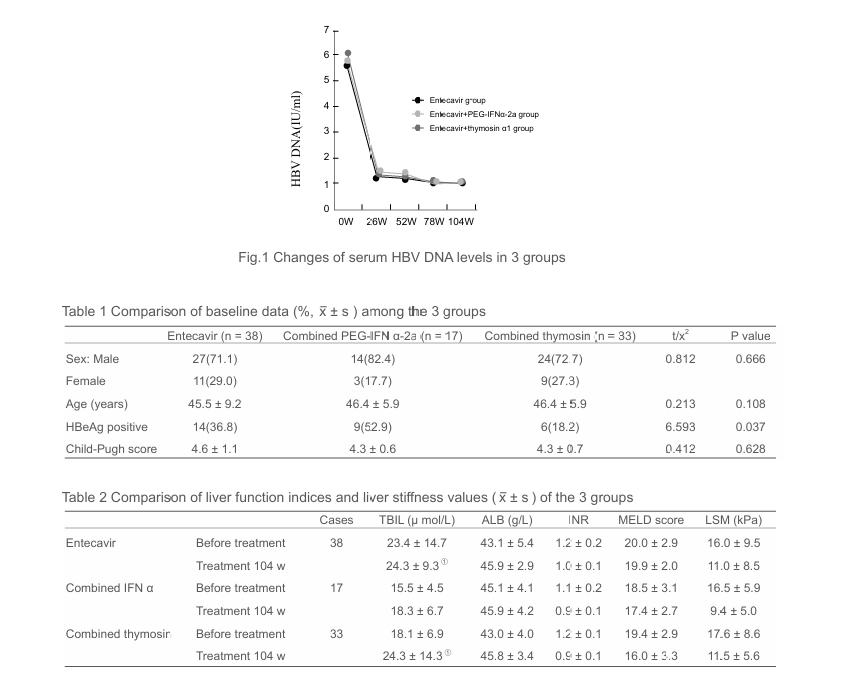
•Serum HBV DNA levels in the entecavir monotherapy group before treatment and at the end of 104 week were (5.6 ± 1.7)IU/ml and (1.0 ± 0.7)IU/ml, respectively (P < 0.05), in PEG-IFNα-2a combination group were (5.8 ± 1.3)IU/ ml and (1.0 ± 0.7)IU/ml, respectively (P < 0.05) and in combined with thymosin group were (6.1 ± 2.0)IU/ml and (1.0 ± 0.9)IU/ml, respectively (P < 0.05), serum albumin levels in the entecavir group before treatment and at the end of 104 week were (43.1 ± 5.4)g/L and (46.9 ± 4.9)g/L, respectively (P < 0.05), and in combined thymosin group were
(43.0 ± 4.0)g/L and (46.8 ± 5.4)g/L, respectively (P < 0.05); there were no significant differences as respect to liver stiffness measures and INRs among the three groups (P < 0.05).
