
•120 patients with hepatitis B induced liver cirrhosis were randomly divided into two groups.
•80 in treatment group receiving compound embryonic bovine liver extract tablets at the base of basic liver protecting treatment; 40 in control group receiving the basic liver-protecting treatment only for 3 months.
•Liver function tests, liver stiffness measurement (LSM) by FibroTouch, and portal vein diameters, spleen thickness and splenic vein width before and after treatment were compared in both groups.
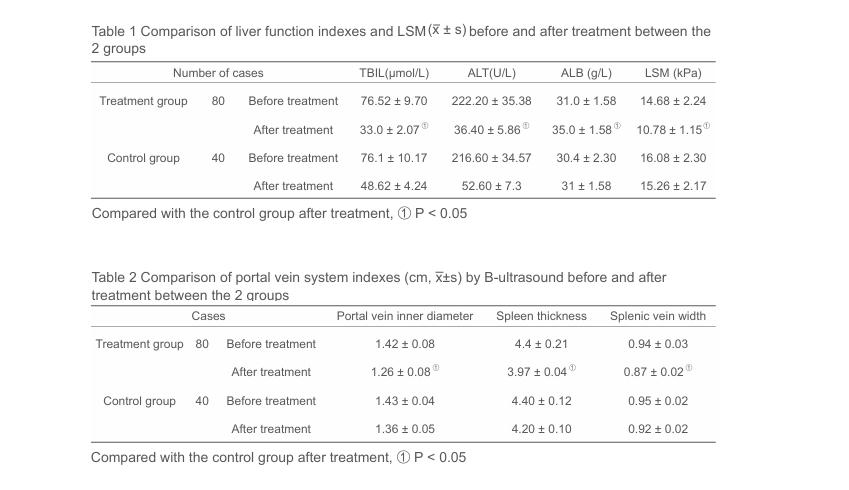
•At the end of 3-month treatment, serum TBIL, ALT, and LSM values in patients in treatment group were (33.0 ±2 .07) μmol/L, (36.40 ± 5.86) U/L, (10.78 ± 1.15) kPa, respectively, significantly decreased as compared with those in the controls [(48.62 ± 4.24) μmol/L, (52.60 ± 7.3) U/L, (15.26 ± 2.17) kPa, respectively, P < 0.05 for all]; serum ALB levels in patients in treatment group increased significantly compared with that in controls [(35.0 ± 1.58) g/L vs.(31 ± 1.58) g/L, P < 0.05].
• Portal vein diameters, spleen thickness and splenic vein widths remarkably decreased compared with those in patients in the control group [(1.26 ± 0.08) cm vs(1.36 ± 0.05) cm, (3.97 ± 0.04) cm vs (4.20 ± 0.10) cm, (0.87 ± 0.02) cm vs. (0.92 ± 0.02) cm, P < 0.05 for all].
